Research projects
Research Projects
Research Support: NIH/NEI (R01EY029709)
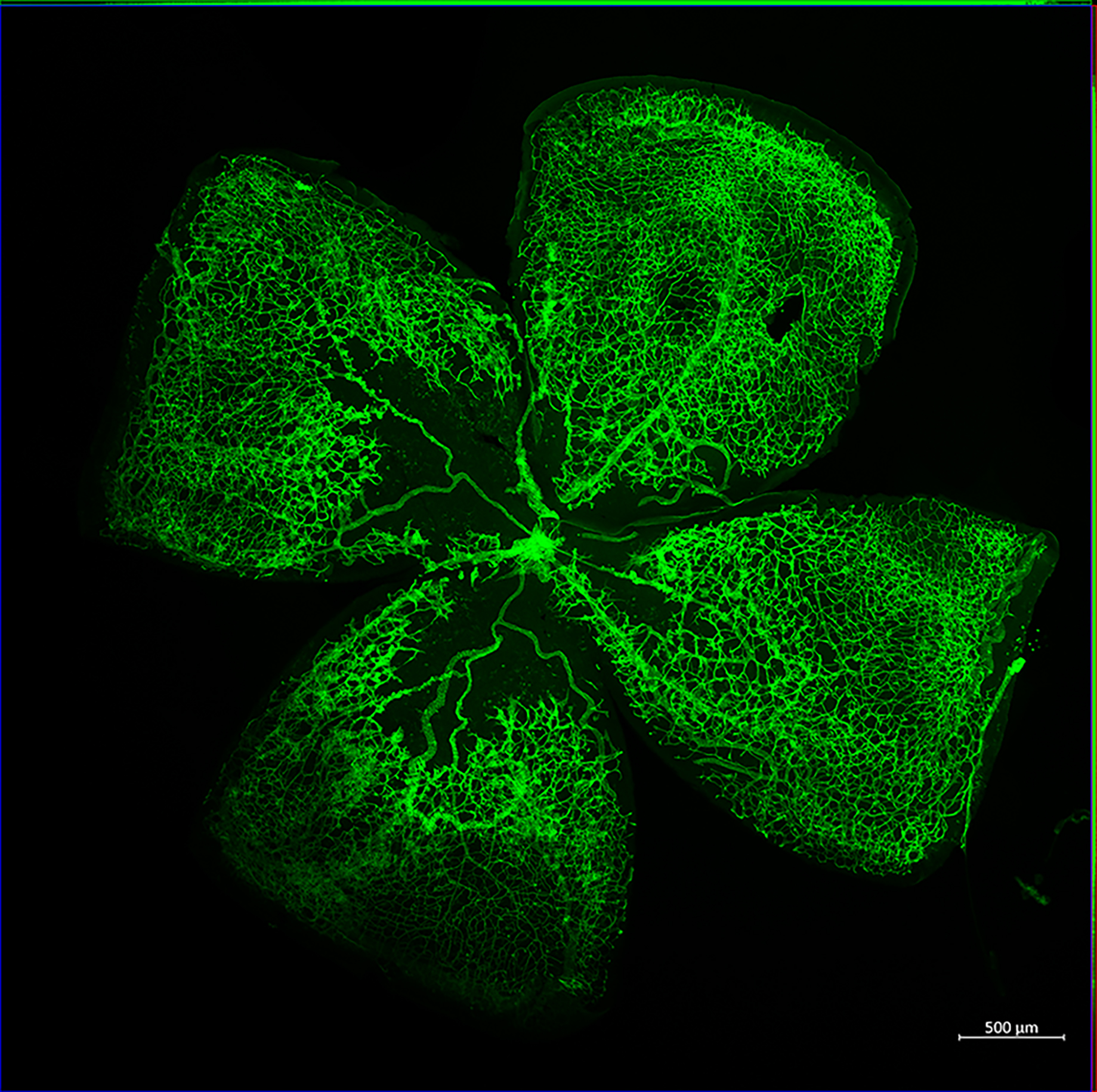
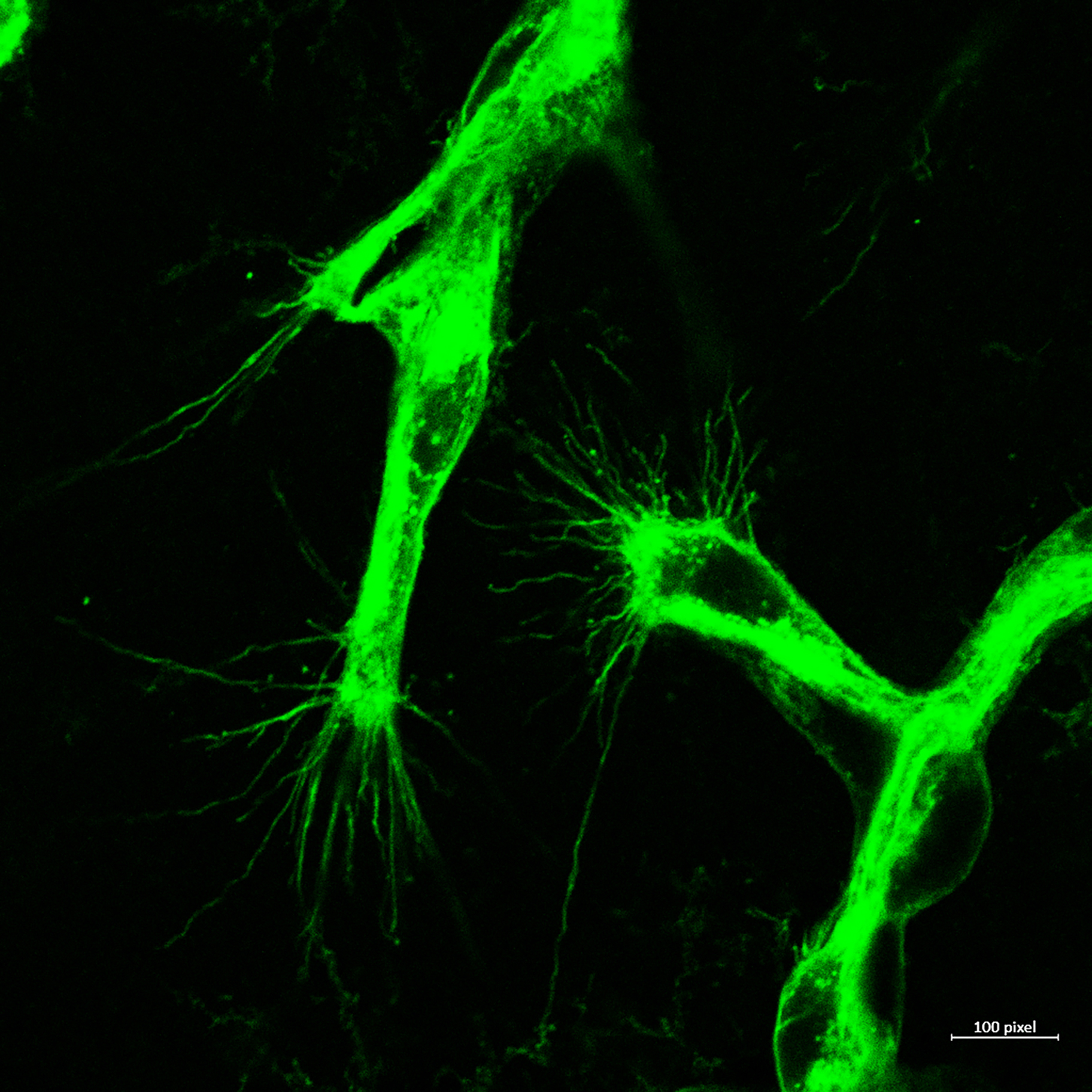
Pathological Retinal Angiogenesis:
Cellular Mechanisms of Pathological retinal Neovascularization: Retinal neovascularization is an ocular manifestation of diabetes, retinopathy of prematurity, and age-related macular degeneration, which leads to vision loss. Despite the use of anti-VEGF and laser treatments, progression of retinal neovascularization continues to cause blindness. The development of new therapeutic approaches against retinal neovascularization is limited, because of lack of knowledge about its pathophysiology. Retinal neovascularization is characterized by production of several angiogenic factors, with consequential growth of aberrant new blood vessels on retinal surface that interferes with light transmission and results in vision loss. An elevated level of inflammation and inflammatory mediators have been observed in retinas or vitreous isolated from patients with pathological retinal neovascularization. Therefore, the ability to modulate inflammation and inflammatory mediators and thereby selectively modulating aberrant retinal neovascularization, would be a great strategy in the treatment of pathological retinal neovascularization. The goal of this study is to not only test the role of IL-33 and caspases in hypoxia-induced pathological retinal neovascularization, but also to understand how these inflammatory molecules regulate sprouting angiogenesis and vessel branching.
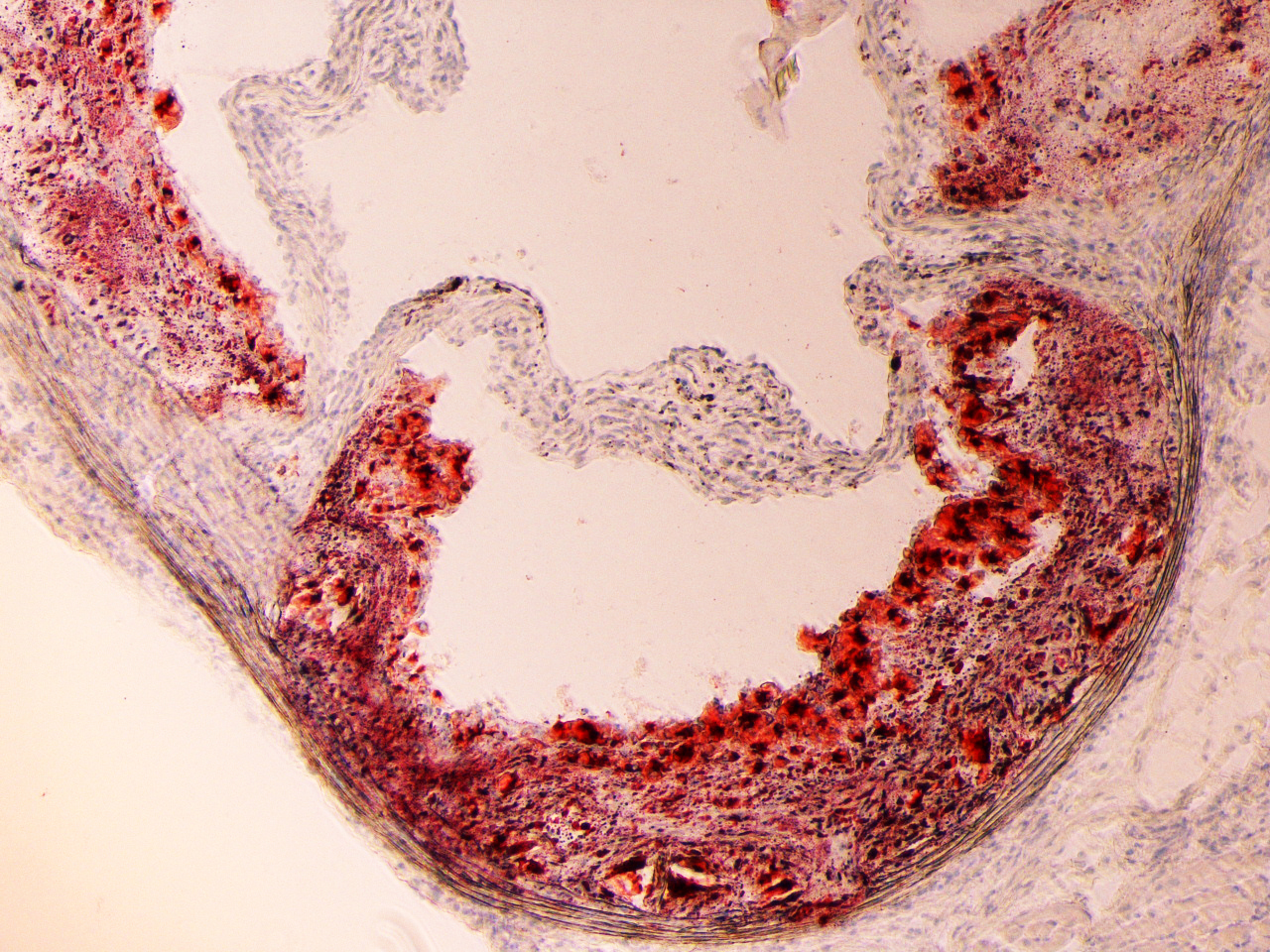
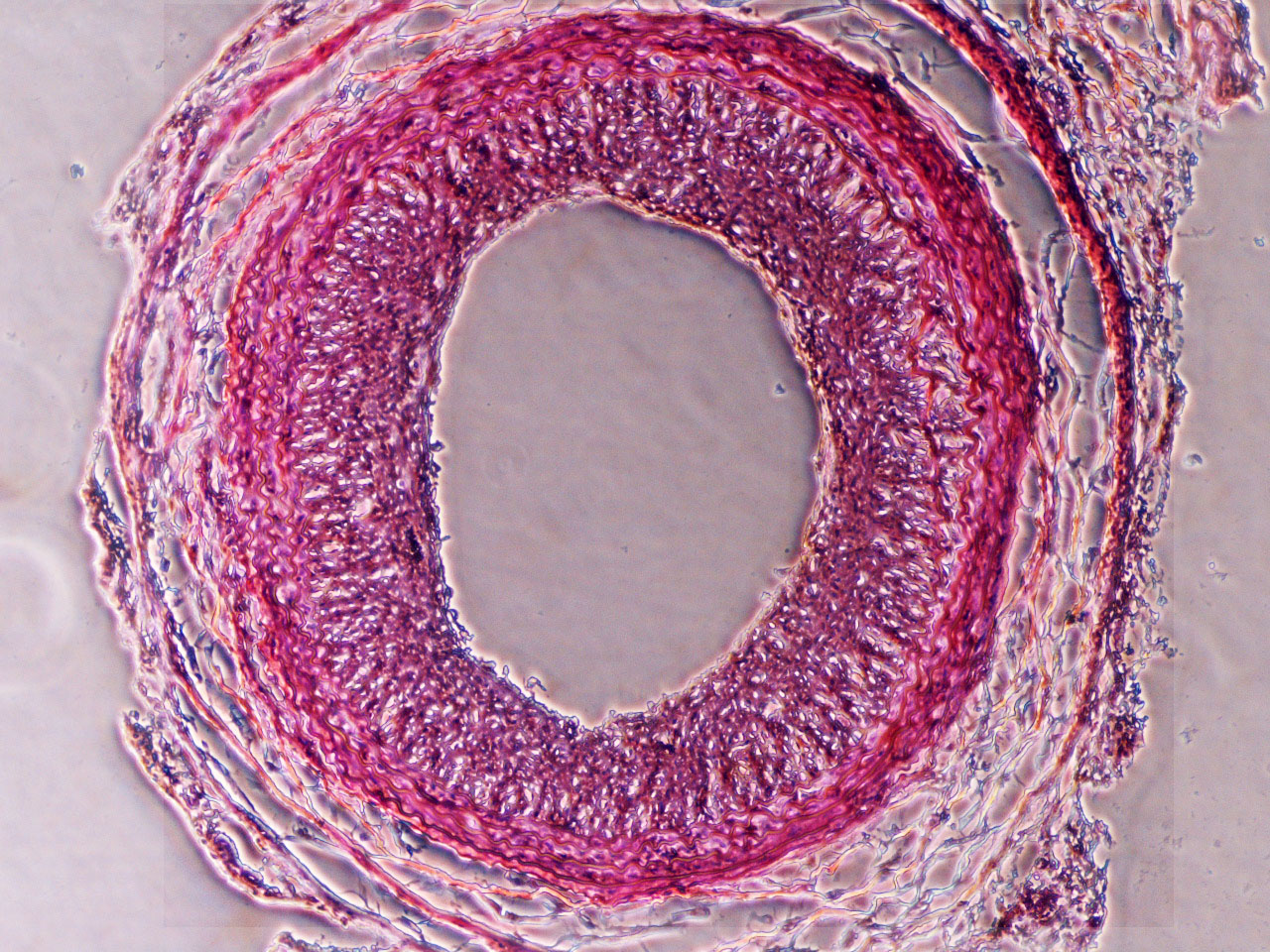
Restenosis/Atherosclerosis:
Regulation of SMC differentiation and migration in restenosis/atherosclerosis: Each year, there are over 7 million cardiovascular bypass and angioplasty procedures performed in the United States. More than one-third of these procedures will have limited durability owing to the formation of intimal hyperplasia, the pathological response of a blood vessel to injury. Although there is no therapy to prevent intimal hyperplasia in bypass grafts, there are some strategies to prevent intimal hyperplasia after angioplasty and stenting. The current antiproliferative agents used to prevent intimal hyperplasia formation inhibit both smooth muscle cell and endothelial cell proliferation. Because these agents prevent the reestablishment of an intact endothelium, patients need to remain on dual antiplatelet therapy indefinitely. An improved strategy to prevent intimal hyperplasia in both surgical bypass and angioplasty and stenting is an unmet clinical need. Pathologically, intimal hyperplasia is a cellmigrationdependent, intimal outgrowth, in which vascular smooth muscle cells (VSMCs) dedifferentiate to a migratory, proliferative, and secretory phenotype. These dedifferentiated VSMCs migrate through the medial layer and invade the intima, where they proliferate and form the hyperplastic neointima. The mechanism by which VSMCs gain motility during intimal hyperplasia development is not fully understood. Our main objective is to target molecules associated with actin and cytoskeleton signaling, that can restrain VSMC translation from contractile to synthetic phase at the initial phase of restenosis.